LITHIUM BATTERIES
Introduction to Lithium-Ion
Lithium-ion batteries were discovered in the 1980s and commercialized in 1991. For the last 10 years, lithium-ion batteries have been used as the main energy storage technology in many different industries. Lithium-ion batteries have many advantages over conventional battery chemistries (lead-acid batteries, nickel-cadmium batteries, gel batteries, etc.). With the advancement in technology, rapid consumption and sustainability moves in today's world, safer and stronger batteries are needed in battery energy storage systems. Lithium element is the best option in terms of energy density. (Wh/Kg) and other added elements, it achieves a very safe and stable structure. Lithium-based batteries and batteries with different chemistry are being developed every day.
What Are Lithium-Ion Batteries?
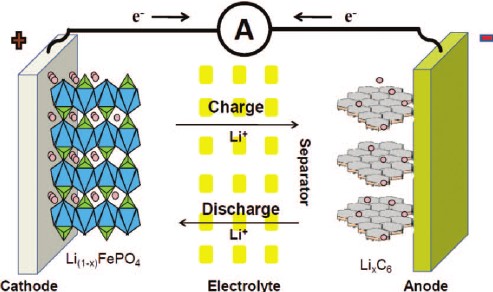
In its simplest form, a lithium-ion cell (battery) consists of a structure that includes a negative electrode (anode) and a positive electrode (cathode) that transfer lithium ions between two materials. Lithium ions move from anode to cathode during discharge and accumulate (intervene) themselves on the positive electrode of lithium and other metals. During charging, this process is reversed.
Lithium-ion batteries (cells) according to their physical structure; It can be cylindrical, prismatic or pouch type. Cells differ in size and shape; some are in plastic and some in aluminum cases. In addition, according to the intended use of lithium-ion batteries; They are differentiated among themselves as energy cells, power cells and conversion cells. Which physical structure and which type of battery will be used depends entirely on the application / project to be made.
.jpg)
Each lithium-ion battery has a safe voltage range in which it can operate. This range depends on the chemistry and application used in the battery. For example, an LFP battery at 0% State of Charge (SOC) is 2.5V and at 100% state of charge (SOC) it is 4.2V. It will be permanently damaged when the voltage of the cell drops below 2.5V and the voltage of the cell is increased above 4.2V. These values are considered the safe operating range of the battery. Going below the specified 2.5V SOC is considered to cause deterioration of the electrodes and over-discharge (deep discharge) of the lithium-ion battery. If a cell is over-discharged repeatedly, it will cause many problems that permanently damage the battery. The same is true for a cell that goes above the specified 100% SOC. These two issues are one of the main items to be considered.
Lithium-ion Batteries, to put it simply; It consists of cell (battery) groups connected in series and parallel with each other and a BMS (Battery Management System). For example, consider an LFP (LiFePO4) cell with a rated voltage of 3.2V and a capacity of 100Ah. Cells cannot be used alone, we need to group cells together to obtain higher voltage and capacity. So how is this done? Cells must be connected in series to increase its voltage. Cells should be connected in parallel to increase capacity. For example, let's say we need an LFP lithium battery with a capacity of 12V / 300Ah; LFP cells will require 3 parallel and 4 serial connections. In this way, we will have a 12.8V / 300Ah LFP lithium battery.
Basic Concepts in Batteries
Anode : The anode is the negative (-) electrode in the cell. It is very common for lithium-ion batteries to consist of lithium and carbon, usually a graphite powder. Thanks to the copper film combined with the electrode, the current can be collected. The purity, particle size and homogeneity of the anode influence its aging behavior and capacity.
Cathode : The cathode is the positive (+) electrode. This is where all the different chemistry comes into play. The cathode is what determines the overall lithium chemistry. Like the anode, it is combined with a current collector material so that electron flow can occur. The cathode is typically combined with an aluminum film. There are many different chemistry as shown above. The main difference between them is the temperature at which they react with the electrolyte (thermal runaway) and the voltages they produce.
Electrolyte : The electrolyte provides the transfer of lithium ions between the plates. Typically, it consists of different organic carbonates such as ethylene, carbonate, and diethyl carbonate. Different mixes and ratios vary depending on the application of the cell. For example, the electrolyte solution for a low temperature application will have a lower viscosity compared to that made for a room temperature environment. Lithium salts are essential in the electrolyte mixture, the salt determines the conductivity of the solution and aids in the formation of the solid electrolyte interface (SEI). In lithium batteries, lithium hexafluorophosphate (LiPF6) is the most common lithium salt. LiPF6 can produce hydrofluoric acid (HF) when mixed with water. SEI is a chemical reaction between lithium metal and electrolyte.
Separator : Lithium-ion cell separators are porous plastic films that prevent direct contact of anode and cathode. The films are usually 20 µm thick and have small spills that allow lithium ions to pass through during the charging and discharging process. A "close" separator is most common. This separator closes the pores to prevent the passage of lithium ions when the cell is out of temperature range or short-circuited. Separators continue to be developed today to improve safety while increasing the capacity of the cells.
Battery Charge Status(SOC) : In lithium batteries, it refers to the remaining capacity in the battery in "percent".
DOD (Discharge of Dept): Indicates how much the lithium battery is discharged in percent.
Cycle Life :It expresses how many times a battery can be fully charged / discharged within the specified criteria.
Energy Density : It expresses the amount of energy that a battery can store in terms of volume and mass. (w/kg , w/cm3)
C rate : It expresses the charge and discharge rate of a lithium battery according to its capacity.
Types of Lithium-Ion Battery Chemistry
Cell chemistry in lithium-based energy storage systems is increasing day by day. However, we can talk about 3 cell chemistry that can be used commercially now and in the next 10 years.
-
Lithium nickel manganese cobalt derivatives (NMC)
-
Lithium iron phosphate (LiFePO4)
-
Lithium titanate (LTO)
LTO, it has a very long life and a wide temperature range. They can handle very high charge and discharge currents. (Rail systems, etc.)
NMC, chemistry used in applications where weight and volume are important. (Electric vehicle, autonomous systems, portable power sources, etc.)
LFP, it is popular in industries with heavy use and harsh environments. It provides a very high cost advantage. (Stackers, renewable energy storage systems, etc.)
Battery Management System (Battery Management System, Battery Control Card)
Lithium is the brain of batteries. Its main task is to protect the battery and to shut down the system by protecting the lithium battery in an unfavorable situation. It controls voltage, current and temperature. It contains communication protocols.